sulfur dioxide lewis structure|SO2(Sulfur Dioxide) Lewis Structure, Hybridization, : iloilo A step-by-step explanation of how to draw the SO2 Lewis Structure (Sulfur Dioxide) Note: From an experimental view (using x-ray crystallography or something similar) we find the single. Jakol Sabay Subo – Ohhh Putangna Ganyan Ang Tipo Ko! Report DL. About. 0 views. 11/08/22. 0%. 0 0. Cowgirl Dirty Talk Dogstyle Instagram Outdoor Public Blowjob Secretary Student Trending Twitter. Related videos. HD 49.78K. 100%. Ganyan Ang Gusto Ko Kristine! Ibuka Mo Pa Punyeta! HD 57.27K. 100%. Sa Banyo Binira Ang .
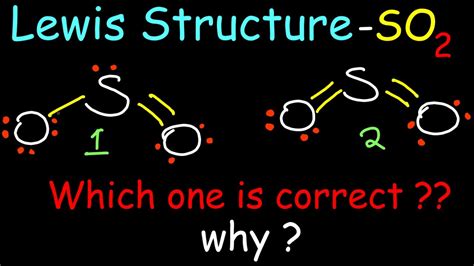
sulfur dioxide lewis structure,How to draw the Lewis Structure of SO2 - with explanationCheck me out: http://www.chemistnate.com This chemistry video tutorial explains how to draw the lewis structure of SO2 also known as Sulfur Dioxide. It discusses the molecular geometry, bond angle, hybridization and formal . The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen.
Learn how to draw the Lewis structure of SO2, a colorless gas with a pungent odor, and its resonance forms. Find out its hybridization, molecular geometry, bond angles, and properties.
A step-by-step explanation of how to draw the SO2 Lewis Structure (Sulfur Dioxide) Note: From an experimental view (using x-ray crystallography or something similar) we find the single.
Learn how to draw the lewis structure of SO2, a pungent-smelling gas with various industrial uses. Find out its hybridization, molecular geometry, and MO diagram with detailed explanations and .Learn how to draw the Lewis structure for SO2, a molecule with a central sulfur atom and two oxygen atoms. Follow the steps to count valence electrons, identify the central atom, connect the atoms, distribute the . To identify the molecular geometry of sulfur dioxide, we must examine its Lewis structure. Two oxygen atoms are linked to the central sulfur atom. There is also a .
By using the lewis concept we can draw the best resonate structure for sulfur dioxide. We can understand the boding between atoms in a SO2 molecule. So .
Example: Consider the Lewis structure for sulfur tetrafluoride (SF 4) which contains 34 valence electrons. SF 4: 6 + 4(7) = 34. . Two Lewis structures can be written for sulfur dioxide. The only difference between these Lewis structures is the identity of the oxygen atom to which the double bond is formed. As a result, they must be equally .
sulfur dioxide lewis structure SO2(Sulfur Dioxide) Lewis Structure, Hybridization, This structure is key to understanding the chemistry of Sulphur Dioxide. We'll. In this video, we'll learn about the Lewis Structure of Sulphur Dioxide - SO2.

The SO 2 Lewis structure depicts the molecular arrangement of sulfur dioxide, which consists of one sulfur atom and two oxygen atoms. In the SO 2 Lewis structure, there is a double bond between the sulfur atom and each oxygen atom. Each oxygen atom possesses two lone pairs, while the sulfur atom has one lone pair. To .SO2(Sulfur Dioxide) Lewis Structure, Hybridization, $\begingroup$ SO2 Lewis Structure - How to Draw the Lewis Structure for SO2 (Sulfur Dioxide) $\endgroup$ – user100905. Commented Sep 17, 2021 at 6:06. . The Lewis structure most closely resembling reality consists of two resonance structures: the first one posted in the question and its mirror image. The reason is that the octet rule is .Example: Consider the Lewis structure for sulfur tetrafluoride (SF 4) which contains 34 valence electrons. SF 4: 6 + 4(7) = 34. . Two Lewis structures can be written for sulfur dioxide. The only difference between these Lewis structures is the identity of the oxygen atom to which the double bond is formed. As a result, they must be equally . SO2 lewis structure consists of Sulphur and oxygen. The chemical formula of sulfur dioxide is SO2. This is color less gas. Smell of SO2 is very much pungent odor. The smell is very much similar to the burnt matchsticks Sulfur dioxide released while volcanic eruptions. SO2 lewis structure shape: The shape of the SO2 is in bent shape. A sulfur atom (S) and two oxygen atoms (O) make up the SO2 Lewis structure. The sulfur atom (S) is the center atom, and the two oxygen atoms (O) surround it at a bond angle of 119 degrees. The sulfur atom (S) and each oxygen atom (O) form two double bonds. The two oxygen atoms (O) each have two lone pairs, while the sulfur . Steps of drawing SO2 lewis structure Step 1: Find the total valence electrons in SO2 molecule. In order to find the total valence electrons in SO2 (sulfur dioxide) molecule, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom. (Valence electrons are the electrons that are present in the . Today in this video, we will determine the Lewis dot structure for sulphur dioxide, having a chemical formula of SO2. It comprises one sulphur atom and two O. The S2O Lewis structure refers to the arrangement of atoms and electrons in a molecule of sulfur dioxide.Sulfur dioxide is a chemical compound composed of one sulfur atom and two oxygen atoms.The Lewis structure helps us understand the bonding and electron distribution within the molecule.In the S2O Lewis structure, the sulfur . The -2 charge means that there are 2 extra electrons. Total: 4 + (3 × 6) + 2 = 24 electrons. The final answer MUST have this number of electrons‼! Step 2) Attach the atoms to each other using single bonds .To draw the Lewis structure for sulfur dioxide (SO2), you first need to determine the total number of valence electrons in the molecule. For sulfur, its valence electron count is 6, and for oxygen, it is 6x2 = 12. Thus, the total is 18 electrons.Sulfur dioxide (IUPAC-recommended spelling) . Structure and bonding. SO 2 is a bent molecule with C 2v symmetry point group. . As a η 1-SO 2 (S-bonded planar) ligand sulfur dioxide functions as a Lewis base .
Lewis structure of SO2 (or Sulfur dioxide) contains two double bonds between the Sulfur (S) atom and each Oxygen (O) atom. The Sulfur atom (S) is at the center and it is surrounded by 2 Oxygen atoms (O). The Sulfur atom has 1 lone pair and both the Oxygen atoms have 2 lone pairs. Let’s draw and understand this lewis dot .

The SO2 Lewis structure would consist of two oxygen (O) atoms and one sulfur atom. Both the sulfur and oxygen atoms have six valence electrons. The molecular geometry of sulfur dioxide is a bent shape. The sulfur to oxygen ratio in sulfur dioxide is 1:2. The sulfur dioxide molecule has two double bonds between the Sulfur atom and .sulfur dioxide lewis structureLewis Dot of Sulfur Dioxide. SO 2. Back. 70 More Lewis Dot Structures. S does not follow the octet rule. It can hold more than 8 electrons. Sulfur having valence electrons in the 3rd energy level, would also have access to the 3d sublevel, thus allowing for more than 8 electrons. NOT IN THIS MOLECULE.Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha. Formal charge on Oxygen = Valence electrons – Nonbonding electrons – (Bonding electrons)/2 = 6 – 4 – (4/2) = 0. So the formal charge on oxygen atom is 0. Now you can see that all the atoms of SO2 have 0 formal charge. This indicates that the overall SO2 (Sulfur dioxide) molecule also has 0 charge and hence it is a neutral molecule.
sulfur dioxide lewis structure|SO2(Sulfur Dioxide) Lewis Structure, Hybridization,
PH0 · Sulfur dioxide (SO2) Lewis Structure, Hybridization
PH1 · Sulfor dioxide: Lewis dot structure for SO2 (video)
PH2 · SO2(Sulfur Dioxide) Lewis Structure, Hybridization, Molecular Geometry
PH3 · SO2(Sulfur Dioxide) Lewis Structure, Hybridization,
PH4 · SO2 Lewis Structure: Drawings, Hybridization, Shape, Charges, Pair And
PH5 · SO2 Lewis Structure: Drawings, Hybridization, Shape, Charges,
PH6 · SO2 Lewis Structure, Hybridization, Molecular
PH7 · SO2 Lewis Structure
PH8 · SO2 (Sulfur Dioxide) Lewis Structure
PH9 · Lewis Structure of SO2 (sulfur dioxide)
PH10 · Lewis Structure of SO2
PH11 · Lewis Structure of SO2